[ad_1]

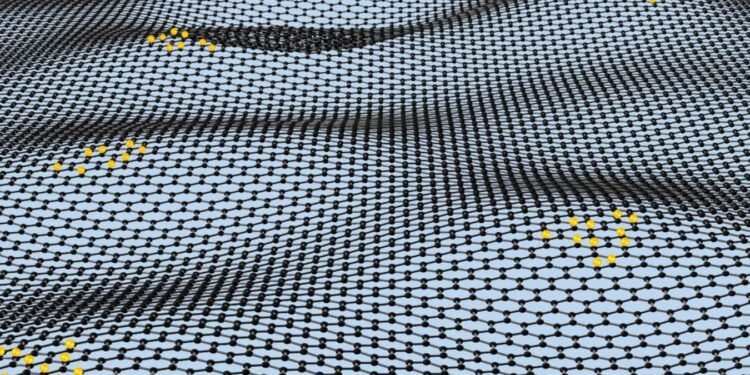
Illustration of nanoripples in sheets of graphene, which make it react effectively with hydrogen (proven in yellow)
Pengzhan
Graphene can break up hydrogen 100 instances higher than any identified chemical catalyst because of tiny ripples on its floor. It may probably be used to develop simpler hydrogen gas cells and make many industrial processes extra environment friendly.
A one-atom-thick layer of carbon, graphene is basically a slice of graphite. The latter is a particularly unreactive compound due to its sturdy carbon bonds.
Nonetheless, Andre Geim on the College of Manchester, UK, and …
[ad_2]
Source link